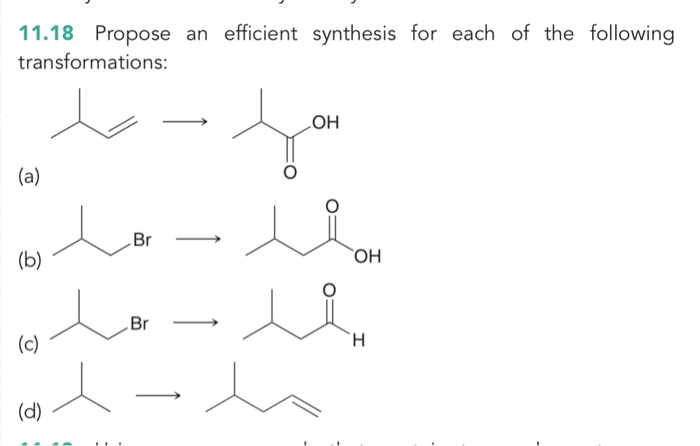
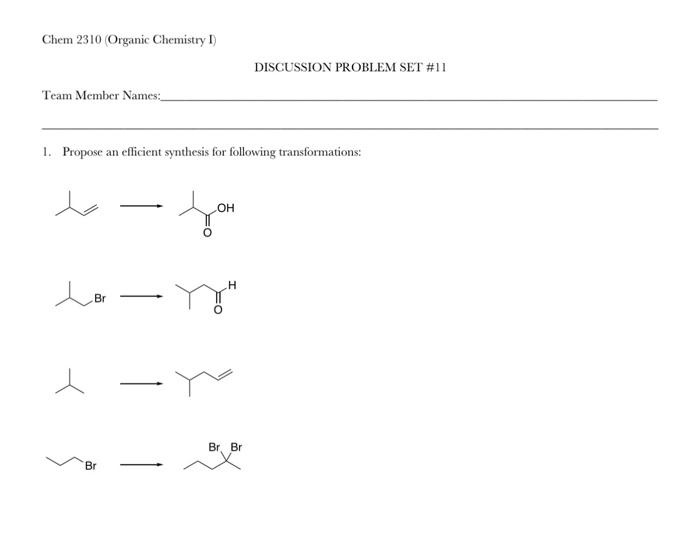
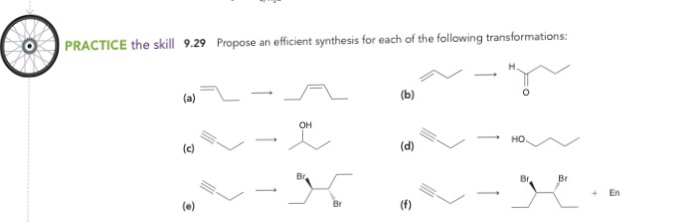
Propose an efficient synthesis for each of the following transformations: This guide delves into the intricacies of organic synthesis, empowering you with a systematic approach to designing and optimizing multistep reactions. By mastering the art of retrosynthesis, reagent selection, and reaction optimization, you’ll unlock the ability to navigate complex transformations with precision and efficiency.
Throughout this comprehensive exploration, we’ll unveil the fundamental principles of functional group chemistry, guiding you through the identification and manipulation of key functional groups. We’ll explore the strategies for optimizing reaction conditions, maximizing yields, and ensuring selectivity. Prepare to embark on a journey that will transform your understanding of organic synthesis and equip you with the tools to conquer even the most challenging transformations.
Propose an Efficient Synthesis for a Given Transformation: Propose An Efficient Synthesis For Each Of The Following Transformations
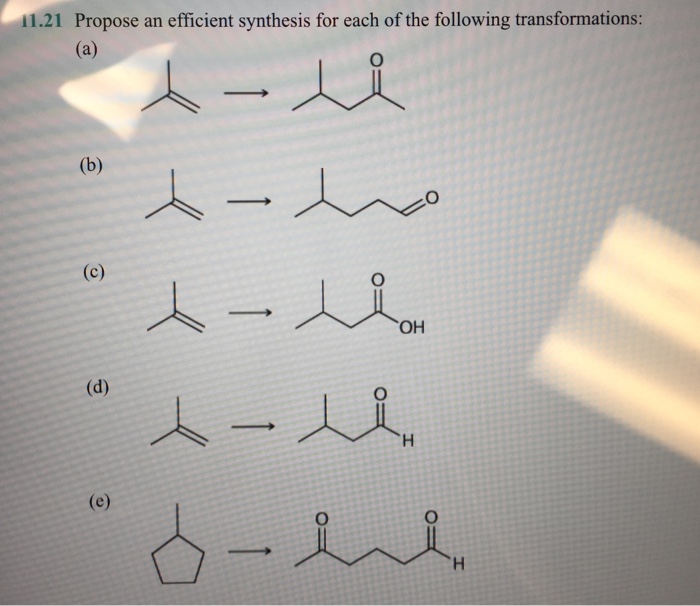
In organic chemistry, the ability to design and execute efficient synthetic pathways is crucial. This article provides a comprehensive guide to propose an efficient synthesis for a given transformation, covering key aspects from functional group identification to reaction optimization.
1. Identify Functional Groups
The first step is to identify the functional groups present in the starting material. Functional groups are specific arrangements of atoms that impart characteristic reactivity and properties to molecules. Common functional groups include alcohols, alkenes, aldehydes, ketones, and carboxylic acids.
Understanding their reactivity helps in selecting appropriate reagents and reaction conditions.
2. Design Retrosynthetic Pathway
Retrosynthesis involves working backward from the desired product to the starting material. It helps identify key disconnections, which are the logical breaking of bonds to simplify the synthesis. By considering the reactivity of functional groups, one can determine the most efficient way to construct the target molecule.
3. Select Reagents and Conditions
Once the retrosynthetic pathway is established, appropriate reagents and reaction conditions must be selected. Factors to consider include selectivity, efficiency, and functional group compatibility. Reagents should be chosen to promote the desired reaction while minimizing side reactions. Reaction conditions, such as temperature, solvent, and catalyst, can significantly impact the outcome of the synthesis.
4. Optimize Reaction Conditions
Optimization of reaction conditions is crucial to maximize yield and selectivity. Variables such as temperature, solvent, and catalyst loading can be adjusted to improve the efficiency of the reaction. Optimization strategies include varying reaction parameters, employing catalysts, and using inert atmospheres to prevent unwanted side reactions.
5. Design Multistep Syntheses, Propose an efficient synthesis for each of the following transformations
Complex transformations often require multiple steps involving several functional group conversions. In such cases, it is necessary to design a multistep synthesis that organizes the steps in a logical and efficient order. Each step should be carefully planned to minimize side reactions and maximize the overall yield of the desired product.
6. Illustrate with Reaction Schemes
Reaction schemes are visual representations of the proposed synthesis. They include all reagents, conditions, and intermediate products. Reaction schemes help communicate the synthetic strategy clearly and concisely, facilitating understanding and reproducibility.
7. Provide Mechanistic Insights
Understanding the mechanisms of the key reactions involved in the synthesis is essential for optimizing the process. Mechanistic insights allow for the identification of potential side reactions and the development of strategies to minimize their occurrence. By considering the role of catalysts, intermediates, and transition states, one can gain a deeper understanding of the reaction pathway.
8. Compare Alternative Approaches
Exploring alternative synthetic routes to the desired product can lead to improved efficiency and selectivity. By comparing different approaches, one can identify the most practical and cost-effective strategy. Factors to consider include the availability of reagents, reaction times, and environmental impact.
Answers to Common Questions
What is the key to proposing an efficient synthesis?
Understanding functional group reactivity, retrosynthetic analysis, and reaction optimization techniques.
How can I optimize reaction yields?
By carefully selecting reagents, reaction conditions, and employing strategies such as temperature and solvent optimization.
What is the importance of retrosynthesis in synthesis design?
Retrosynthesis allows you to break down complex transformations into simpler steps, guiding your reagent and condition selection.